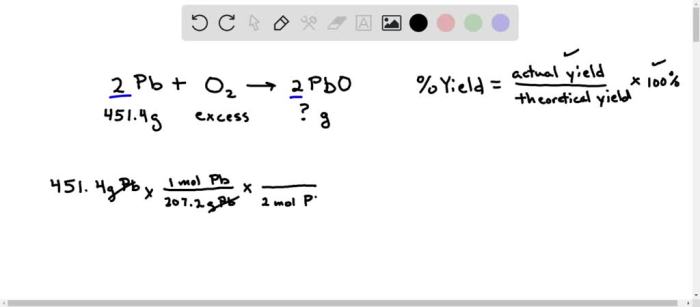
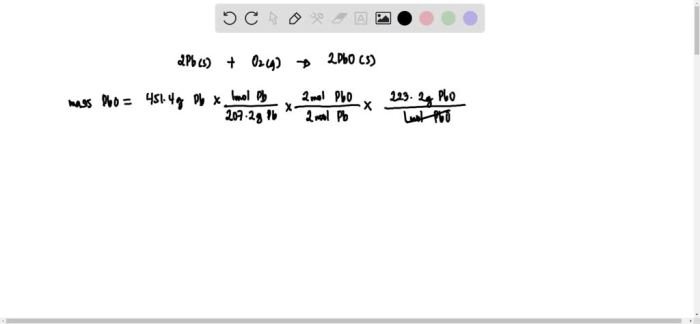
An excess of oxygen reacts with 451.4 g of lead, setting the stage for an intriguing exploration of chemical reactions and their implications. This detailed investigation delves into the intricacies of this reaction, examining the reactants, products, stoichiometry, and limiting reactants.
Moreover, it explores the reaction conditions, potential applications, and the significance of excess oxygen in this process.
As we embark on this scientific journey, we will uncover the fascinating details of this reaction, providing a comprehensive understanding of its mechanisms and applications.
Chemical Reaction
When excess oxygen reacts with lead, it undergoes a chemical reaction to form lead oxide.
The balanced chemical equation for this reaction is:
Pb(s) + 3O2(g) → 2PbO(s)
Reactants and Products
- Reactants:Lead (Pb) and oxygen (O 2)
- Products:Lead oxide (PbO)
Lead is a soft, malleable metal with a silvery-white appearance. Oxygen is a colorless, odorless gas that makes up about 21% of the Earth’s atmosphere.
Lead oxide is a yellow or reddish-brown powder that is insoluble in water. It is used in the production of glass, ceramics, and batteries.
Stoichiometry
To calculate the moles of lead used in the reaction, we divide the given mass by its molar mass:
Moles of Pb = 451.4 g / 207.2 g/mol = 2.18 mol
According to the balanced chemical equation, 2 moles of lead react with 3 moles of oxygen. Therefore, the moles of oxygen that reacted with the lead are:
Moles of O2= (2.18 mol Pb) × (3 mol O 2/ 2 mol Pb) = 3.27 mol
Mass-to-Mass Relationship, An excess of oxygen reacts with 451.4 g of lead
To convert the moles of oxygen to grams, we multiply by its molar mass:
Mass of O2= (3.27 mol O 2) × (32.00 g/mol) = 104.6 g
The calculated mass of oxygen (104.6 g) is less than the given mass of lead (451.4 g). This indicates that oxygen is the limiting reactant in this reaction.
Limiting Reactant
The limiting reactant is the reactant that is completely consumed in a chemical reaction, limiting the amount of product that can be formed.
In this reaction, oxygen is the limiting reactant because it is present in a smaller molar ratio compared to lead. This means that all of the oxygen will be consumed before all of the lead is used up.
Excess Reactant
The excess reactant is the reactant that is present in a larger molar ratio than the limiting reactant.
In this reaction, lead is the excess reactant. It is present in a larger molar ratio than oxygen, which means that some of the lead will remain unreacted after the reaction is complete.
Reaction Conditions
The reaction between excess oxygen and lead occurs at room temperature and pressure. It is a slow reaction that can be accelerated by heating or by the presence of a catalyst.
Applications
The reaction between excess oxygen and lead has several applications, including:
- The production of lead oxide, which is used in the production of glass, ceramics, and batteries.
- The removal of lead from contaminated soil and water.
- The production of lead-based pigments.
FAQ Resource: An Excess Of Oxygen Reacts With 451.4 G Of Lead
What is the balanced chemical equation for the reaction between excess oxygen and lead?
2Pb + 3O2 → 2PbO
How many moles of lead are used in the reaction?
To determine the moles of lead, we divide the given mass (451.4 g) by the molar mass of lead (207.2 g/mol): 451.4 g / 207.2 g/mol = 2.18 moles
What is the limiting reactant in this reaction?
Lead is the limiting reactant because it is completely consumed in the reaction, while oxygen is in excess.