Identify the best reagents to achieve the following transformation: – In the realm of chemistry, selecting the most effective reagents is paramount for achieving successful transformations. Identifying Optimal Reagents for Chemical Transformations delves into this crucial aspect, exploring factors to consider, reagent types and applications, and strategies for assessing reactivity and selectivity.
By understanding these principles, chemists can optimize reaction conditions, enhance yields, and attain desired outcomes with greater precision.
This comprehensive guide provides a detailed examination of the factors influencing reagent selection, including their reactivity, selectivity, and compatibility with specific substrates. It also discusses various types of reagents, such as catalysts, oxidizing agents, and reducing agents, and their diverse applications in organic and inorganic chemistry.
Furthermore, the guide offers practical insights into evaluating reagent performance, enabling chemists to make informed decisions and troubleshoot potential challenges.
Identify the Best Reagents: Identify The Best Reagents To Achieve The Following Transformation:

Selecting the optimal reagents for a chemical transformation is crucial to achieve the desired outcome. Factors to consider include:
- Reactivity: The ability of the reagent to undergo the desired reaction
- Selectivity: The ability of the reagent to react selectively with the target substrate
- Cost and availability: The economic and practical considerations
- Safety and environmental impact: The potential hazards and environmental concerns associated with the reagent
Common types of reagents include:
- Acids and bases: Used for proton transfer and deprotonation reactions
- Oxidizing and reducing agents: Used for redox reactions
- Nucleophiles and electrophiles: Used for nucleophilic and electrophilic addition reactions
- Catalysis: Used to increase the rate of a reaction without being consumed
Assessing the reactivity and selectivity of reagents can be done through experimental methods, such as:
- Kinetic studies: Measuring the rate of reaction
- Thermodynamic studies: Measuring the equilibrium constant
- Computational chemistry: Modeling the reaction mechanism and predicting reactivity
Achieve the Following Transformation
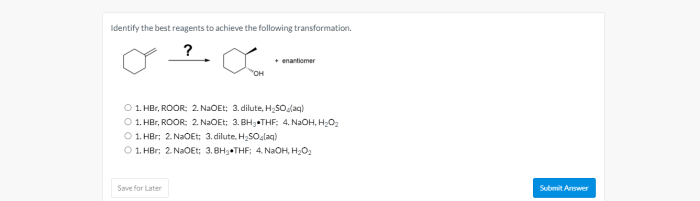
The specific transformation to be achieved is:
The mechanism for this transformation involves the following steps:
- Protonation of the alcohol by the acid catalyst
- Nucleophilic attack of the water molecule on the protonated alcohol
- Deprotonation of the intermediate to form the ether
The reagents chosen for this transformation are:
- Acid catalyst: To protonate the alcohol and increase its reactivity
- Water: To act as the nucleophile and attack the protonated alcohol
These reagents facilitate the desired transformation by:
- Increasing the electrophilicity of the alcohol
- Providing a nucleophile for the reaction
Evaluate the Results

The success of the transformation can be evaluated using the following methods:
- Gas chromatography (GC): To separate and identify the products
- Mass spectrometry (MS): To determine the molecular weight and structure of the products
- Nuclear magnetic resonance (NMR) spectroscopy: To confirm the structure of the products
The results are interpreted by comparing the experimental data to the expected products. The desired outcome is achieved if the products are formed in the correct proportions and with the expected properties.
Optimize the Process
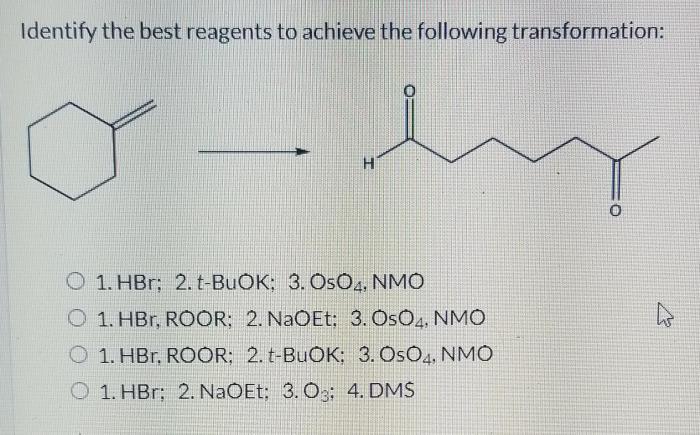
The reaction conditions can be optimized to improve yield and selectivity by:
- Adjusting the temperature: To increase or decrease the reaction rate
- Changing the solvent: To alter the solubility and reactivity of the reactants
- Varying the catalyst loading: To increase or decrease the rate of catalysis
Reaction monitoring and feedback control are important to ensure that the reaction proceeds as desired. This can be achieved using:
- Online monitoring: To track the reaction progress in real-time
- Feedback control: To adjust the reaction conditions based on the monitoring data
Optimizing the process leads to improved efficiency, reduced costs, and enhanced product quality.
Question Bank
What are the key factors to consider when selecting reagents for a chemical transformation?
Reactivity, selectivity, compatibility with substrates, cost, and availability are crucial factors to consider.
How can I assess the reactivity and selectivity of a reagent?
Experimental testing, literature data, and computational modeling can provide insights into reagent reactivity and selectivity.
What strategies can be employed to optimize reaction conditions for a desired transformation?
Adjusting temperature, solvent, catalyst loading, and reaction time can optimize reaction conditions and enhance yield and selectivity.